The importance of alcohol in perfume is a mandatory phenomenon. It acts as a solvent to help diffuse fragrances. Alcohol in perfumes enhances their longevity. The odor performance of perfumes also becomes better and intense.
This blog explores various types of alcohol used in perfumes. It explores the different roles alcohol performs in perfumery. It explains the impact of alcohol on the perfume quality.
What is the Source of Alcohol in Perfume?
Before diving into the why and what of the concept of “alcohol in perfumes,” let’s know more about the source of alcohol. Where does alcohol come from?
The most common type of alcohol in perfume is ethanol or ethyl alcohol. Ethyl alcohol comes from natural sources. Corn fermentation is the natural way of obtaining ethyl alcohol. The fermentation process is further followed by purification and distillation. We shall discuss the process in detail later in this blog.
The primary sources of alcohol are carbohydrates, including starch from sugar, potatoes, and grains. However, the alcohol used in perfume manufacturing is usually derived from raw food materials.
Apart from natural synthesis, another way is to make alcohol in a laboratory. Keep in mind that whether produced naturally or artificially, the final properties of ethanol or alcohol remain unchanged.
Bioethanol is another component of natural perfumes. Its source is vegetables, which is why it is also known as agricultural ethanol.
What are Alcoholic Perfumes?
“You may be wondering why alcohol is used in perfume?”
or
“Does perfume have alcohol in it?”
Of course yes
Alcohol is a primary ingredient of perfumes.
Alcoholic perfumes, also known simply as perfumes or Eau de parfum (EDP), are scent mixtures that utilize Alcohol (specifically ethanol) as the primary solvent.
There are various compositions in perfumery, including Alcohol. The primary components of this type of fragrance are the concentrated scent mixture and Alcohol. These components help distribute the scent and reveal the fragrance’s complete aroma profile.
Due to its lack of smell and neutrality, this component, called ethyl alcohol or ethanol, won’t alter the fragrances detected by the aroma concentrate. Numerous regions have restrictions on using perfumes with Alcohol and only allow them for cosmetic purposes. Adhering to regulations ensures that these products are both practical and safe.
Role of Alcohol in Perfumery?
Alcohol is a key ingredient in perfumes. The role of alcohol in perfume is a mandatory process.It works well because it can store and transfer aromatic oils. The presence of Alcohol in perfume aids in the uniform dispersal of the aromatic compounds, which results in the gradual release of the fragrance over time.
Alcohol in fragrance acts as a solvent to help dissolve and stabilize the aromatic compounds. It also contributes to the volatility of the fragrance by assisting in the evaporation of the top notes, which are the first fragrances you detect upon spritzing the perfume.
Solvent
In the distillation of fragrance concentrate, alcohol acts as a strong base.The scent oils and additional ingredients in the scent are transported or dissolved in Alcohol. In most cases, the solvent or alcohol is ethyl alcohol or ethanol. The concentrate, on the other hand, consists of raw materials such as flowers, spices, herbs, and wood. Alcohol aids in spreading the aromatic compounds by evenly and effectively dissolving and mixing them.
Alcohol as a Fixative
Although alcohol itself is not a fixative yet, it does play the role of a fixative in perfumery.
How? Certain fixatives, such as smooth, creamy sandalwood, and green vetiver, are added to perfumes along with alcohol. These fixatives help in the gradual disbursement of fragrance.
Volatility
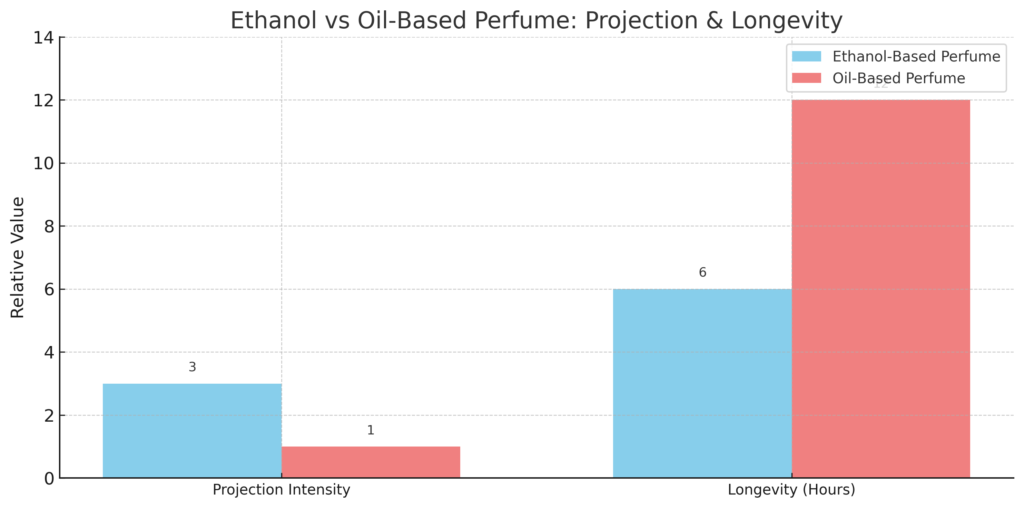
The Alcohol in the perfume causes it to evaporate faster once it is sprayed onto the skin. This volatility releases fragrance molecules into the air, creating the desired scent effect. A 2022 study in Fragrance Science Quarterly found that ethanol-based perfumes evaporate 3x faster than oil-based alternatives, ensuring top notes are instantly perceptible (Source: FSQ, Vol. 12).It is also non-toxic to the skin and spreads evenly.
Stability
Alcohol keeps the perfume formula stable by inhibiting the growth of germs. It does this by maintaining the scent’s purity over time. It also helps in the preservation of fragrance duration.
Fragrances containing Alcohol remain highly favored in the market. This is due to their effectiveness and longstanding history of usage. These scents are ideal for making a strong statement and emphasizing your personality with a distinctive scent.
Fragrances with Alcohol have a more excellent projection and leave a more potent scent trail because of the higher alcohol content, leading to a more robust and noticeable scent. This intense fragrance will attract attention and help you make a statement as you move through a group.
Scent’s Duration and Strength
Can perfumes with alcohol last longer?
Of Course yes
Alcohol in perfume determines the strength and duration or longevity of perfume. You might have noticed that perfumes differ based on alcohol concentration. Eau de parfum has a high density of natural elements and a low density of alcohol. Eau de cologne, on the other hand, has a high density of alcohol in it.
Types of Alcohol and their Sources
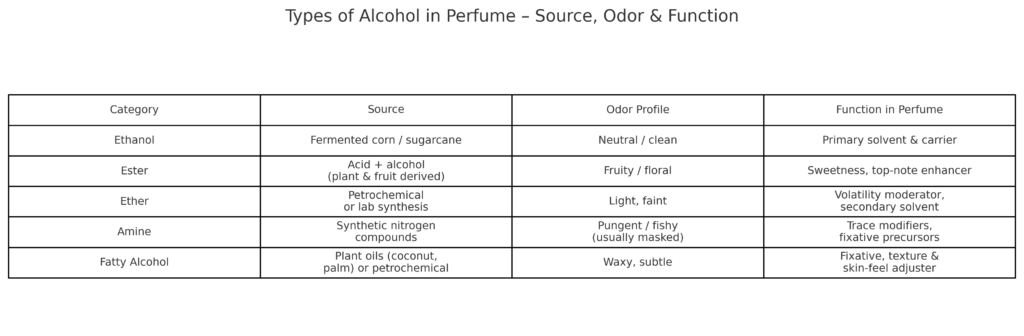
Amine
Amines are not often directly utilized in perfumery because of their firm and usually disagreeable smells, such as fishy or resembling ammonia. Some amine derivatives, specifically those connected to particular chemical groups, can be created to generate fragrant molecules. These substances are cautiously employed to add unique nuances or underlying scents to perfumes, creating a variety of aroma characteristics.
Alcohol
In the fragrance industry, “alcohol” typically denotes ethanol, which is widely utilized as a solvent in perfumes. Ethanol is a vehicle for fragrance oils and other components, aiding their dispersion and penetration into the skin. Due to its rapid evaporation, ethanol assists in the diffusion of the fragrance and enhances its projection. Ethanol plays a crucial role in determining the volatility and potency of the perfume.
Ester
Esters are commonly utilized in the fragrance industry due to their pleasant aromas, often fruity or floral—they form into acid and Alcohol. Esters contribute to the sweetness, freshness, or depth of scents and the indent in the top, middle, and base notes. Whereas benzyl acetate emits a floral perfume, ethyl acetate has a fruity aroma.
Ether
Although fragrance makers rarely use ethers because of concerns regarding their stability and diminished odor intensity, ethers are frequently used in perfumes as solvents or dilution agents to control the evaporation rate and aroma dispersion. Ethers are responsible for maintaining a perfume’s overall aroma and durability.
Ethers are generally lighter and have lower water solubility and boiling points than alcohols. Due to their low reactivity, they are used as solvents for fats, oils, waxes, fragrances, resins, dyes, gums, and hydrocarbons.
Ethanol
Ethanol, also known as ethyl alcohol or grain alcohol, is frequently used in the creation of perfumes. It combines well with absolutes, fragrance oils, and other aromatic components.
Ethanol is required to dissolve and combine essential oils to manufacture perfumes. Its rapid evaporation achieves dry skin. Applying it to the skin gives the impression of coolness. Its crucial role in the fragrance industry is to provide an even distribution of aroma particles and facilitate skin application.
Production methods of Ethyl Alcohol

Ethanol or ethyl alcohol is the most commonly used solvent or alcohol in perfume. It is produced using two methods: fermentation and synthetic production. The fermentation process takes place in six steps.
Fermentation Method:
Fermentation is a biological process in which microorganisms convert sugars into alcohol and carbon dioxide. It is the primary process for producing ethanol for industrial purposes.
Steps in the Fermentation Process
Selection of Raw Materials
Raw materials for fermentation are all natural. These include corn, potatoes, molasses, etc.
Saccharification
Saccharification is the process of breaking down complex molecules into simple sugars by using enzymes. In fermentation, saccharification breaks down complex starches in wheat and potatoes into simpler sugars.
Fermentation
This step involves introducing yeast into the sugar solution, which typically consists of glucose and fructose. The yeast breaks down the sugar solution into ethanol and carbon dioxide. This time-consuming process takes 24 to 72 hours, and a temperature of 25-35 degrees Celsius has to be maintained during the process.
Distillation
Distillation is the process of separating ethanol from water. It is necessary because fermentation creates mash, a mixture of ethanol and water, along with other impurities.
Dehydration
The resulting ethanol from distillation further undergoes dehydration to obtain ninety-nine percent pure ethanol.
The Effect of Alcohol Concentration on Perfume
Alcohol in perfume comes in a range of strengths, influencing their intensity and lastingness:
| Eau de Cologne: | This product contains 2-5% fragrance oils and a higher alcohol content (70-80%). It offers a light and refreshing scent suitable for daily wear. |
| Eau de Toilette: | It contains 5-15% fragrance oils and a moderate alcohol content (80-90%). Compared to Eau de Cologne, it provides a more pronounced scent that lasts longer. |
| Eau de Parfum: | This type of perfume contains 15-20% fragrance oils and a lower alcohol content (80-90%). It delivers a richer and more intense fragrance experience and is often chosen for evening wear. |
| Perfume (Extrait de Parfum): | This type contains the most concentrated fragrance oils (15-40%) and a lower alcohol content (70-92%). It offers the most potent and long-lasting fragrance experience, requiring only a tiny amount for a strong effect. |
Fragrances containing Alcohol are well-known for their capacity to produce intricate, elegant, and long-lasting scents. They create a blend that lingers on the skin and makes a strong impact. Alcoholic perfumes have a strong presence in the fragrance industry. They offer diverse scents to cater to different preferences and occasions.
Choosing the Right Perfume based on Alcohol Concentration:
Now that you know the various types of perfumes based on alcohol concentration it is easier for you to make an informed decision. You can purchase a perfume of your choice based on various alcohol concentration in perfume. For example If you prefer a light, everyday fragrance, choose Eau de Cologne, which has a higher alcohol concentration; if you’re looking for a long-lasting, rich scent, Eau de Parfum is a better choice. If you are interested in less alcohol content than choose Extract de Parfum.
Use of Denatured Alcohol in Perfumes
What is denatured alcohol in perfume?
The answer is simple i.e the alcohol that is not fit for drinking.
Denatured alcohol is a perfume concentrate. It has a strong smell and a very bitter taste. This alcohol is not fit for human consumption, and therefore, it has no connection with alcoholic beverages such as vodka. Basically, the use of denatured alcohol in perfumes helps the manufacturers avoid the excise duty they have to pay on alcoholic beverages.
Making of Denatured Alcohol:
The basic purpose of making denatured alcohol is to make it unfit for human consumption. For this purpose, manufacturers use additives. Various additives make alcohol unfit for drinking. The addition of chemicals alters its taste, smell, and even color.
Is Denatured Alcohol safe in Perfume?
A lot of consumers question the use of denatured alcohol in perfumes. The main question is about its safety. Is denatured alcohol safe for use in perfume?
Yes it is
The alcohol denat in perfume is always an excellent concentrate or solvent, and it keeps its chemical properties unchanged. If you read the perfume labels, you will often find the term “perfume denat” written on them.
Alternatives to Non- Alcoholic Perfumes
Yes, the very first perfumes were non-alcoholic ones called ‘attars.’ These strong scents came from the Middle East and India, where they were created by distilling plants like flowers, spices, and herbs and then combining them with essential oils like sandalwood.
Oil-based perfumes, which are classified as alcohol-free scents, are still available today and may be sold by contemporary manufacturers in rollerball or dropper forms. They are gaining more popularity, especially among individuals with sensitive skin or those who favor alcohol-free products. These perfumes do not contain ethanol, commonly used as a solvent and carrier in traditional perfumes.
Non-alcoholic perfumes use alternative solvents or carriers instead of Alcohol, such as:
Oil-based formulas: Perfumes that use jojoba oil, fractionated coconut oil, or other natural oils as a base.
Water-based formulas: Perfumes are diluted with water, or water-based emulsions are used to distribute the fragrance.
“Oil-based perfumes” or “water-based perfumes” are fragrances without Alcohol. They don’t dry out the skin like alcoholic fragrances; people like their gentleness. They also have a distinct feel when applied, setting them apart from conventional alcohol-based scents.
Non-alcoholic perfumes were created for several reasons. Individuals who are allergic to Alcohol or any of its constituents or who follow strict religious guidelines could choose an alcohol-free fragrance. Alcohol-free perfumes contain very little to no alcohol, which lessens the impact on the skin and lowers the chances of causing irritation or allergic reactions.
Alcohol-free fragrances have been developed to provide an alternative for those who find traditional perfumes with Alcohol too strong or irritating for their skin. This allows individuals to enjoy the art of perfume-making without the potential drawbacks of Alcohol, offering a personalized and enjoyable scent experience. In addition, specific individuals favor non-alcoholic fragrances for their ability to provide a more delicate, personal aroma that lingers closer to the body.
Impact of Alcohol in Perfume on Shelf Life
Alcohol in perfume does have a strong interfuse; ‘s s of life. The relationship between alcohol in perfume and its shelf life is proportional to each. But why?
The reason is apparent. As mentioned earlier, perfumes with 80% ethanol content show 50% slower oxidation rates compared to non-alcoholic variants, extending shelf life to 5+ years (IFRA, 2023).Sometimes, perfumes may change their color and scent. If this happens, then you should stop using the perfume ASAP. To avoid such circumstances, perfumes usually have a PAO (Period after opening) label on the bottles and boxes. This label has a symbol that includes a jar with an open lid. This indicates the period the perfume remains suitable for use once the lid has been opened.
Conclusion
Alcohol has been used for centuries in the synthesis of scents. From ancient royal settings to modern perfume factories, alcohol is widely used. Alcohol helps perfumes last longer by dissolving precious oils and extracts. It adds depth to the fragrance and balances it.
Alcohol is essential for a perfume to achieve improved dispersion, longevity, and overall sensory impact. According to perfume manufacturers, it is versatile and great for making intricate, enduring scents that attract a wide range of customers.

