Everybody is aware of the comprehensive range of electroplating applications in various industries. Have you ever wondered why this process is so widely used at industrial level?
Electroplating has the ability to combine the useful properties of various metals together. The addition of copper can increase electrical conductivity. Gold and silver enhance the aesthetics.
Several metals have unique characteristics. Electroplating provides an opportunity to combine the positive properties of other metals together onto one base metal.
This blog explains the process in detail with its advantages and limitations.
Metal Plating Finishes
Metal finishing solutions or plating finish is a comprehensive yet creative process. It involves the coating of a metal on the outer surface of a material. The metals can be nickel, chromium, or copper.
The basic purpose of metal finishing is very simple. It is to provides corrosion resistance. Also, to beautify the product and increase its aesthetic appeal.
Metal finishing services on metals and materials provide them with a lot of benefits. Especially in case of machined parts.
Metal finishing improves the surface of the part and makes it smooth. It decreases the surface roughness. Metal finishing makes the material stronger and harder and offers better adhesion of paint.
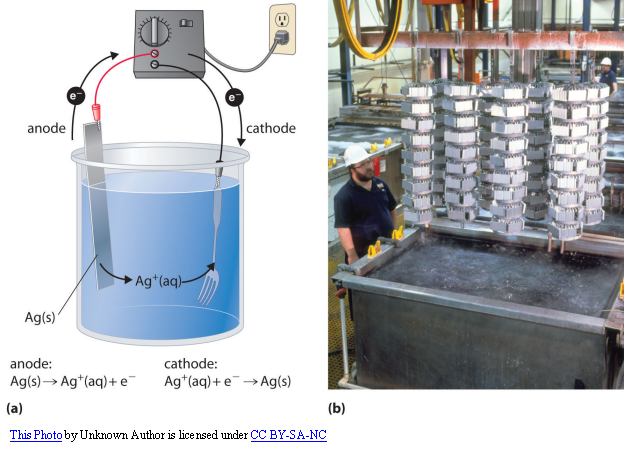
What is the metal plating process?
The metal plating construction process involves a series of steps which are as follows:
Removing Harmful contaminants
The first step is about cleaning up. This removes harmful contaminants from the substrate. This step is important because the contaminants can hinder the electroplating process. Cleaning and ringing are part of this step.
Determine the effectiveness of the cleaning process
Determination of effectiveness of cleaning process is important. Different treatments have different cleaning requirements. It all depends upon the type of the substrate and the result you are expecting. In some instance you need simple cleaning of soil. In more complex instances you might also need to remove oil and grease.
Setting up the Station
Setting up the complete electroplating station is important. The important components are:
- Anode
- Cathode
- Electrolyte
- Current supplying battery
Process Execution
The plating process starts when you pass current through the station. The thickness of the coating metal depends upon various factors. These are:
- The duration of electric current.
- The intensity or voltage of electric current.
- Temperature
The duration of immersion of plates in the electrolyte or submersion tank.
Post Cleaning
Once the process is complete you need to do cleaning again. This is known as post treatment cleaning as it occurs after the completion of process and surface treatments.
Disposing the Waste
Waste disposal is important in electroplating process It is because this process produces heavy metal disposals which are hazardous to the environment.
Types of Metal Plating Process
There are two types of metal plating process. These are electroplating and autocalyctic. These two types differ from each other on the basis of use of electric current. Electroplating uses electricity for execution. Autocalyctic process does not require the use of direct electric current either.
Electroplating
Also known as electrolytic plating, this process causes a plating solution by reduction of metal ions by passing electric current that causes a chemical reaction. This chemical solution is called an electrolyte. In this process the executioners have complete control over the electroplating process.
Autocalyctic
Also known as electroless plating this process uses non-conductive substrates. The metal atom reduction is actually induced by the chemical reaction. It is however difficult to have control over this process. Another disadvantage is that the chemical bath in this case has a limited lifetime.
Types of Metal Plating Finishes
Zinc Plating
Do you why zinc is among the most desirable metals for coating. It is because it is inexpensive and easily available. Zinc plating can be implemented through following processes:
Electroplating
The mechanism of the electroplating process is very simple. The substrate (the material to be electroplated) acts as the cathode. Pure zinc plate acts as the anode. Zinc oxide solution acts as the electrolyte.
On passing electric current thick layer of zinc particles coats the material. You can control the thickness of coating by increasing or decreasing the electric current. Zinc coating is galvanized and ductile in nature.
Electroplating process is also named as cold process. When the material to be coated is zinc the name of the process becomes zinc plating.
Molten Bath
Molten bath dipping is a very simple process. You need zinc in molten form. Simply by dipping the substrate you can achieve zinc coating. In case of haphazard shapes addition of small amount of aluminum improves the fluidity of, molten zinc. This improves the quality of coating.
In the same way addition of small amount of tin can improve the metal and surface finishing.
Metal Spray
This technique is also known as metalizing. In this technique zinc or any other metal that needs to be coated is present in the form of a wire or metal powder. We use a flame to melt the powder or wire. Later we impinge the molten metal on the metal surface of the substrate.
The surface coatings in this case are the result of the mechanical bond between the metal and the substrates. Metal spray is a useful technique if you need to achieve thick coating.
Cadmium Plating
Another metal used in the electroplating process is Cadmium. Cadmium plating refers to the coating of cadmium on a substrate through electroplating. It forms a soft metallic silver coating on the base metal.
Cadmium plating is mostly used in the electroplating of automotive parts. It can also be used in place of zinc as an alternate. Cadmium had this amazing ability of being water resistant. That is why it is most suitable meetable to be used in marine industries.
The use of cadmium in aircraft industry is also very prominent. It is because parts with cadmium plating are durable. They require less repairing.
Chrome Plating
This is one of the most desirable industrial plating services. It because of many reasons. It is hard and it offers high corrosion resistance. At the industrial level chrome plating is known as hard chrome plating. It is because industries need parts with extremely high corrosion resistance and hardness.
The main reason behind this high corrosion resistance is that in hard chrome plating three layers of metals are coated over each other. It is done on nickel. Nickel is already coated over iron. Therefore, the three coating layers over the base metal increase the resistance of the materials to corrosion.
Nickel Plating
When considering electroless nickel plating is the first element that comes in mind. As discussed before nickel is the underlying coating metal in chrome plating but they also bond with titanium, copper and aluminum. Household fixtures such as shower heads, door knobs and other materials use nickel plating.
Electroless nickel plating is very simple. We use nickel phosphorous alloy for plating purposes. The low and even high percentage of phosphorous adds to quality characteristics. In high percentage phosphorous provides high resistance. When in low quantity it provides high magnetism.
Copper Plating
When looking for cost effective plating techniques and metals copper is the first metal that comes to mind. The reason is quite simple. It offers high level of conductivity and is very easy on pocket.
In most of the cases copper plating is the preliminary step before coating of the actual metal. Its use is common in electrical parts and appliances. It is because of the high conductivity it offers. Plating companies that are looking for high plating efficiency metal in less cost should definitely consider copper.
Gold Plating
Depositing a thin layer of gold on the base metal refers to gold plating. Gold is a precious metal. It has a conductive surface. It also offers high resistance to oxidation.
The most extensive use of gold plating is in the manufacturing of gold-plated jewelry. Metallic jewelry that already has copper or silver coating on it goes through gold plating. Another important use is in case of electrical connectors. In case of electrical connectors, it improves the electrical conductivity.
In case of gold plating there is a disadvantage of tarnishing with time. However, this can also be reduced. A single strike of nickel on gold plating can make it resistant to the threat of being tarnished.
Silver Plating
Silver is among the decorative metals. Therefore, silver plating is used to enhance the aesthetic appeal of metal products. When gold plating is not affordable and you are looking for cheaper alternative than silver plating is the best option. Silver is cheaper than gold. It also offers high electrical conductivity.
The only limitation is that silver is not resistant to humidity unlike gold. It is galvanic in nature. When exposed to humid environment it corrodes.
Tin Plating
Tin is the most commonly used metal in food processing and storage industries. You may have noticed that vast array of food packages is made of tin. It is because tin provides excellent protection against harmful effects of external environment.
In case of food packaging the walls of the containers are first greased with food oil. This process refers to passivation. A unique feature of tin sheets is that they can have variable thickness in the same container. The inner layer can be thin and the exterior layer can be thick. It depends upon the environmental conditions they are facing.
Terneplate Technique for Roofing
Terneplate is another type of tin plating. This technique is used to provide corrosion protection to steel. It uses an alloy of tin and lead. This technique is widely used in roofing industries. With proper maintenance it can last up to ninety years.
However, in the modern era tin plating is done over stainless steel. It no more combines with lead to make the alloy.
Rhodium Plating
Another type of metal plating finish that gives a white lustrous appearance to base metal is rhodium plating. Rhodium is a type of platinum. With silver, copper and platinum as base metals it can give a shiny appearance to noble metal with tarnish resistance.
However, the exposure of harsh environment might cause the rhodium layer to lose its shine and luster. It might require replating after few years.

What is the difference between Powder Coating and Metal Plating?
Although both are finishing processes there are still many differences. The main difference is in their execution.
Powder Coating
As the name suggests powder coating involves the coating metal in the form of a free flowing powder. This powder is simply applied on the surface of the substrate.
Later the administration of UV lights or flame cause the coating of the powder on the surface of the substrates. It is an electrostatic process. Also, it is a dry process since o liquid is involved.
The resulting coating from powder coating acts as an adhesive paint.
Metal Plating
As described before metal plating is a wet process. It is impossible without the administration of electric current and electrolytic solution. The resulting coating from metal plating does not appear as a paint. It appears as a metallic finish instead.
ADVANTAGES OF ELECTROPLATING
Corrosion Resistance
The most important advantage of electroplating is that it makes the base metal corrosion resistance. It improves the resistance of the metal to harmful effects of the external environment.
However different metals have different levels of resistance. Some might be more resistant and others might offer fewer resistant properties.
Decorative purposes
Another major advantage of electroplating is its use in decorative purposes. Consider the example of chrome plating services. They provide a reflective finish that makes their use suitable in furniture manufacturing.
Industries such as the automotive sectors and jewelry take maximum advantage of the decorative abilities of electroplating process.
Enhanced Durability
The subsequent layers of metals one above the other increase the durability of the base metal. The more the layers the more durable the metal is. This application is most useful in automotive and hardware industries. It is because the mechanical processes in these industries go through a lot of high pressure and stress.
Increased Conductivity
All the metals in electroplating such as silver, copper, gold etc. offer high electrical conductivity. Therefore, electroplating on base metals increase their conductivity further. This application is useful in electrical industries. Telecommunications and aerospace industries also exploit this application.
Reduction of Friction
Another unique advantage of electroplating is increase in lubrication. The subsequent metallic coatings decrease friction between moving parts and increase their lubricity.
LIMITATIONS OF ELECTROPLATING PROCESS
Although there are plenty of advantages of electroplating process yet it also has certain limitations.
The major limitation is the production of hazardous waste material. Pretreatment before disposal can reduce the toxicity of the waste.
Workers working around the electroplating station are exposed to hazardous materials and chemicals. This poses a major health risk to them. Use of precautionary equipment can reduce this limitation.
Conclusion
Electroplating comes with a comprehensive range of advantages and limitations. Yet it is a widely used process both at small and industrial level. The metal finishing produced from electroplating has applications in versatile industries.
With right expertise and precautionary measures industrialists can take maximum advantage of this technique. You can also outsource the process to a third-party company that has expertise in this technique.



